Key Takeaways
-
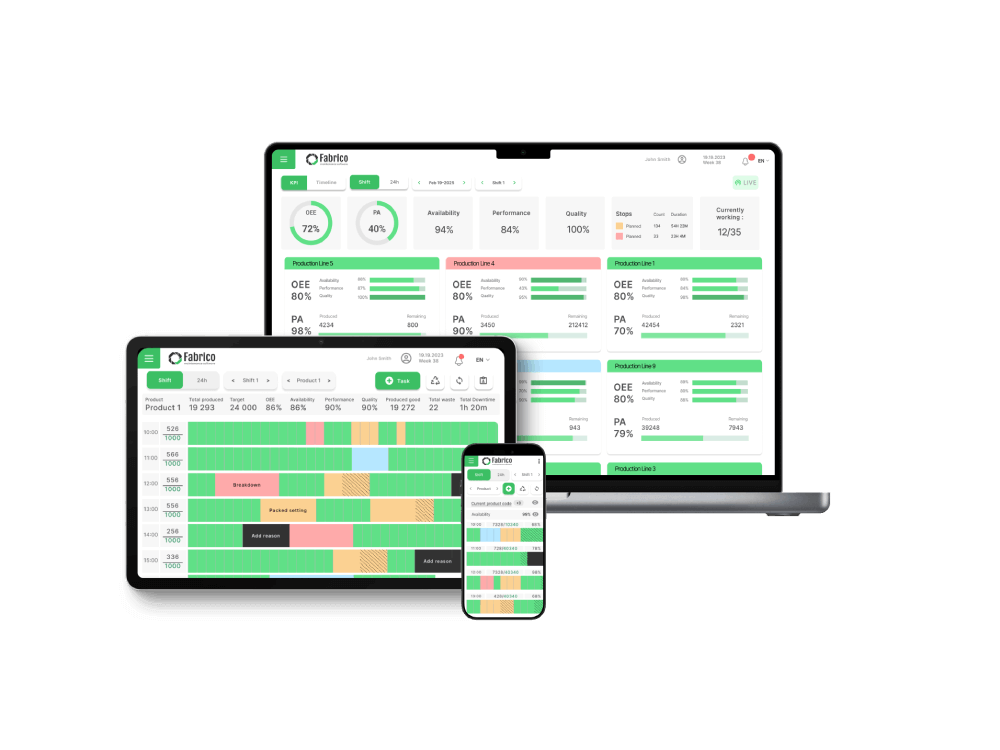
In pharma, OEE is a critical tool for both improving efficiency and strengthening GMP (Good Manufacturing Practices) compliance.
-
The biggest challenge is ensuring data integrity for audits. This requires a system with automated data collection (including Computer Vision for legacy lines) that meets 21 CFR Part 11 requirements.
-
A true pharma solution doesn't just diagnose a deviation with OEE; it uses an integrated CMMS to manage the auditable CAPA (Corrective and Preventive Action) and maintenance response.